LINK ALCOHOL ,PHENOL AND ETHER CONCISE NOTES

ALCOHOL PHENOL ETHER
1. Account
for the following:
a. C – O – H bond angle in alcohol is less than tetrahedral angle.
b. C – O bond length in phenol is shorter than that in methanol.
c. C – O – C bond angle is greater than the tetrahedral angle.
d. The boiling points of alcohols and phenols are higher than
corresponding alkanes of same molecular mass.
e. Among the isomeric alcohols the boiling point follows the order 30 < 20 < 10.
f. Lower alcohols are soluble in water
g. Ethanol is less acidic than methanol.
h. The acidic character of the alcohols follows the order 10 > 20 > 30
i. The reaction of alcohol with acid is carried out in presence of
small amount of concentrated H2SO4.
j. Reaction of alcohol with acid chloride is carried out in presence
of a base pyridine.
k. Ortho and para isomers of nitrophenol can be separated by steam
distillation.
l. Phenol is ortho-para directive to electrophilic substitution.
m. The reaction of an alkoxide with a tertiary alkyl halide gives an
alkene.
n. Alkyl aryl ethers give phenol with HI instead of iodobenzene.
o. Aralkyal ethers (or aryl alkyl ethers) are ortho para directing.
p. Glycerol is used in cosmetics.
q. Orthonitrophenol is more acidic than orthomethoxyphenol.
2. Effect
the following conversions:
Chloro benzene to phenol
Propene to 1-Propanol
Propene to 2-propanol
Benzene sulphonic acid to phenol
Phenol to toluene
Acetone to 30 butyl alcohol
Ethanol to isopropyl alcohol
Phenol to anisole
Aniline to phenol
Phenol to picric acid
Phenol to aspirin
Cumene to phenol
Phenol to p-hydroxy acetophenone
Ethene to glycol
CO to methanol
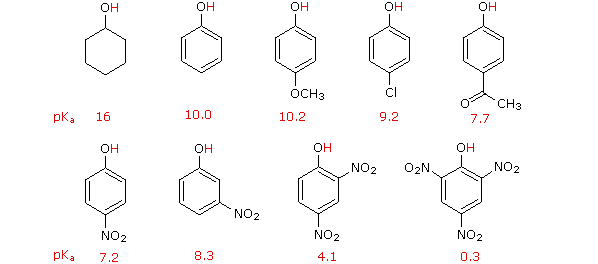
3. Arrange the following on the increasing property given in bracket:
a. Pentan-1-ol, butan-1-ol, butan-2-ol, ethanol, propan-1-ol, methanol
(Boiling Point)
b. Pentan-1-ol, n-butane, pentanal, Ethoxyethane (Boiling point)
c. Propan-1-ol, 2, 4, 6 – trinitro phenol, 3,5 – dinitro phenol,
4-mehylphenol (Acidity)
d. Ter. Butyl alcohol, isobutylalcohol, n-butyl alcohol (Acidity)





.jpg)







What are Alcohols Phenols and Ethers
ReplyDeleteThese three categories of organic compounds are frequently employed in a wide range of commercial and personal applications. But what are they exactly?
Alcohol is formed when a saturated carbon atom joins with a hydroxyl (-OH) group.
Phenol is formed when the -OH group replaces the hydrogen atom in benzene.
Ether is formed when an oxygen atom joins two alkyl or aryl groups.
Classification of Alcohols Phenols and Ethers
Nomenclature
Preparation of Alcohols
Preparation of Phenols
Preparation of Ethers
Because this blog post focuses mostly on the chapter, it is not necessary to read the whole chapter from the textbook. The subtopics addressed in Alcohols Phenols and Ethers for NEET notes are as follows: